New article published on the Journal of Fungi by Luca Spaggiari, Gabriele Tedeschi, Giulia Benatti, Michael De Benedittis, Maria Teresa Franzè, Diego Pinetti, Eva Pericolini and Andrea Ardizzoni.
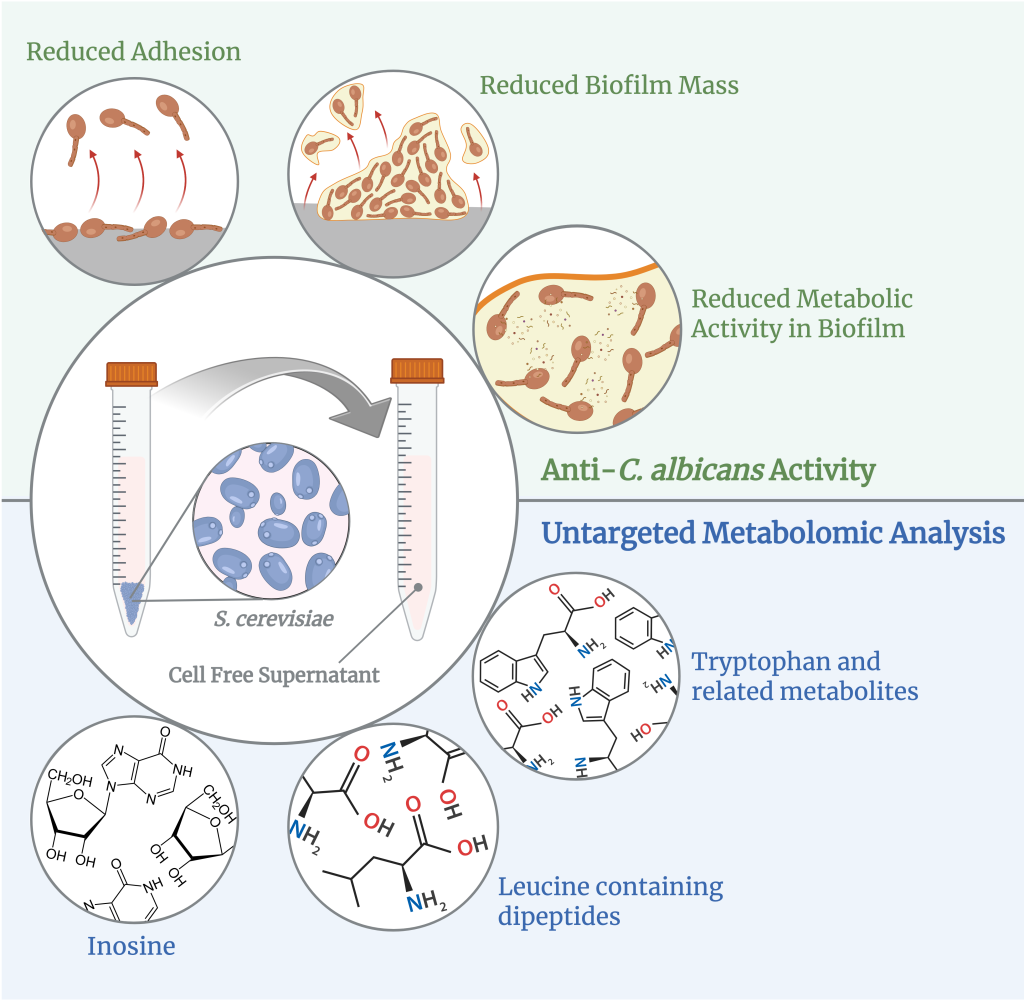
This study presents an untargeted metabolomic analysis of cell-free supernatants derived from various clinical isolates of the yeast Saccharomyces cerevisiae and investigates their effects on the opportunistic fungal pathogen Candida albicans, known for its clinical relevance in human infections.
The results provide novel insights into the metabolic profiles of S. cerevisiae supernatants and explore their potential antifungal properties against C. albicans, contributing to our understanding of yeast-microbe interactions and opening avenues for future applications in fungal biology and therapeutic research.
Read the full open-access article here: https://www.mdpi.com/2309-608X/12/2/81
ABSTRACT
Saccharomyces cerevisiae probiotic properties are effective for the treatment of infections by the opportunistic pathogen Candida albicans. Here, we assessed the anti-Candida effect of cell-free supernatants (CFSs) from three different fecal isolates and one ATCC strain of S. cerevisiae. We evaluated C. albicans growth inhibition through CFUs, and the impairment of virulence factors (adhesion, biofilm formation, and metabolic activity) by crystal violet and XTT assays. An untargeted metabolomic analysis of the CFSs was also performed. The CFSs moderately reduced C. albicans growth, but they could impair C. albicans virulence by reducing its capacity to adhere and to form a biofilm, and by decreasing the metabolic activity of biofilm-embedded fungal cells. The untargeted metabolomic analysis indicated an overexpression of N-acetyl-DL-tryptophan and other molecules derived from its metabolism (kynurenic acid and indole-3-acrylic acid), the dipeptides glycyl-L-leucine, prolyl-leucine, and γ-L-glutamyl-L-leucine, and the unconventional nucleotide inosine in the CFSs from fecal isolates, as compared to the reference strain. Further studies are warranted to better characterize the metabolome of these CFSs. Should the effects described here also be confirmed in vivo, the possible future employment of S. cerevisiae CFSs as a postbiotic aid to the current antifungal therapy may be considered.